- MGC Pharmaceuticals (MXC) has issued almost 3300 prescriptions of its standardised cannabinoid medicines in Australia, U.K., Ireland, and Brazil
- This represents a 64 per cent increase in prescriptions since January
- As a result of this increase, MGC Pharma will begin rolling out its expanded Mercury MGC Pharma product line to include six extra products with higher percentages of CBD and THC
- MGC Pharma is up a steady 4.35 per cent and shares are trading for 2.4 cents each
MGC Pharmaceuticals (MXC) has issued 3295 prescriptions of its standardised cannabinoid medicines in Australia, U.K., Ireland, and Brazil.
This represents a 64 per cent increase in prescriptions since January.
The majority of these prescriptions were CannEpil, MXP100, and MP100, and an average of 35 per cent of repeat patients received two or more prescriptions.
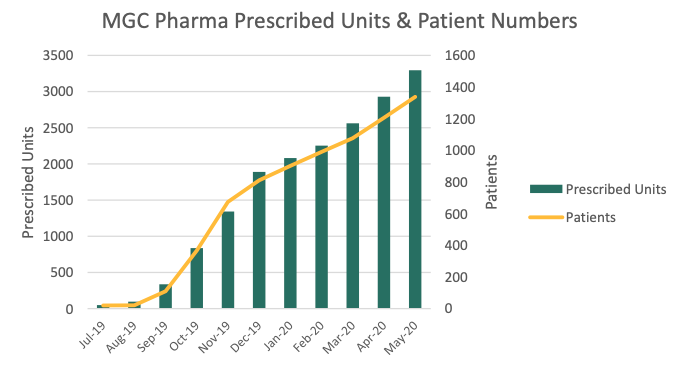
Further, since its first patient received medication of CogniCann in mid-January, 50 prescriptions have been issued to 23 patients.
Both of these achievements represent a significant milestone for MGC Pharma given that higher than expected sales have been received despite COVID-19.
As a result of these increases, MGC Pharma will begin rolling out its expanded Mercury MGC Pharma product line to include six extra products with higher percentages of CBD (cannabidiol) and THC (tetrahydrocannabinol) to exiting markets.
The company is confident that this expansion will be well received given the strong pipeline for its exisiting products.
Based off recent evaluations, MGC Pharma has ended its product distribution exclusivity agreement with GrowBiotech to allow it to focus on new sales and distribution partnerships in the U.K.
“The company is extremely pleased to have achieved the strong growth in both prescription and patient numbers in what has been a challenging operating environment due to the outbreak of COVID-19,” Co-Founder and Managing Director Roby Zomer commented.
“The company is responding to the current strong order pipeline and we look forward to further sales growth opportunities with the expansion of the Mercury MGC Pharma line of products,” he added.
A review of scheduling low-dose CBD, which will be considered at the Australian Therapeutic Goods Administration (TGA) Joint Advisory Committee in June, may result in some CBD medicines being reclassified to a lower classification.
This will allow low-dose CBD products to be purchased at pharmacies without a prescription.
MGC Pharma is up a steady 4.35 per cent and shares are trading for 2.4 cents each at 3:16 pm AEST.







