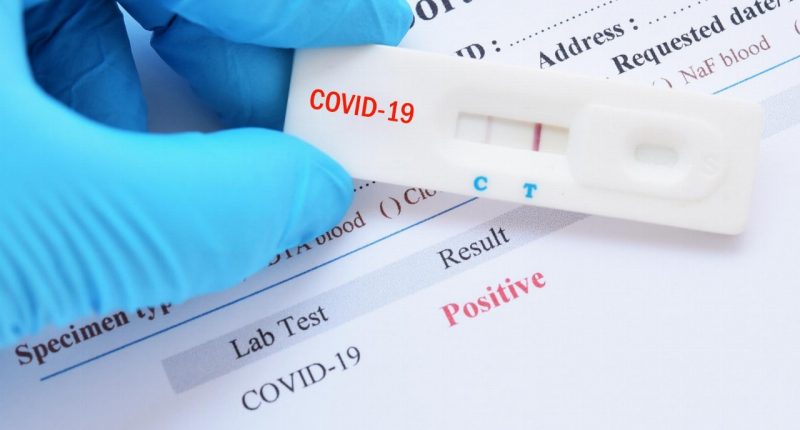
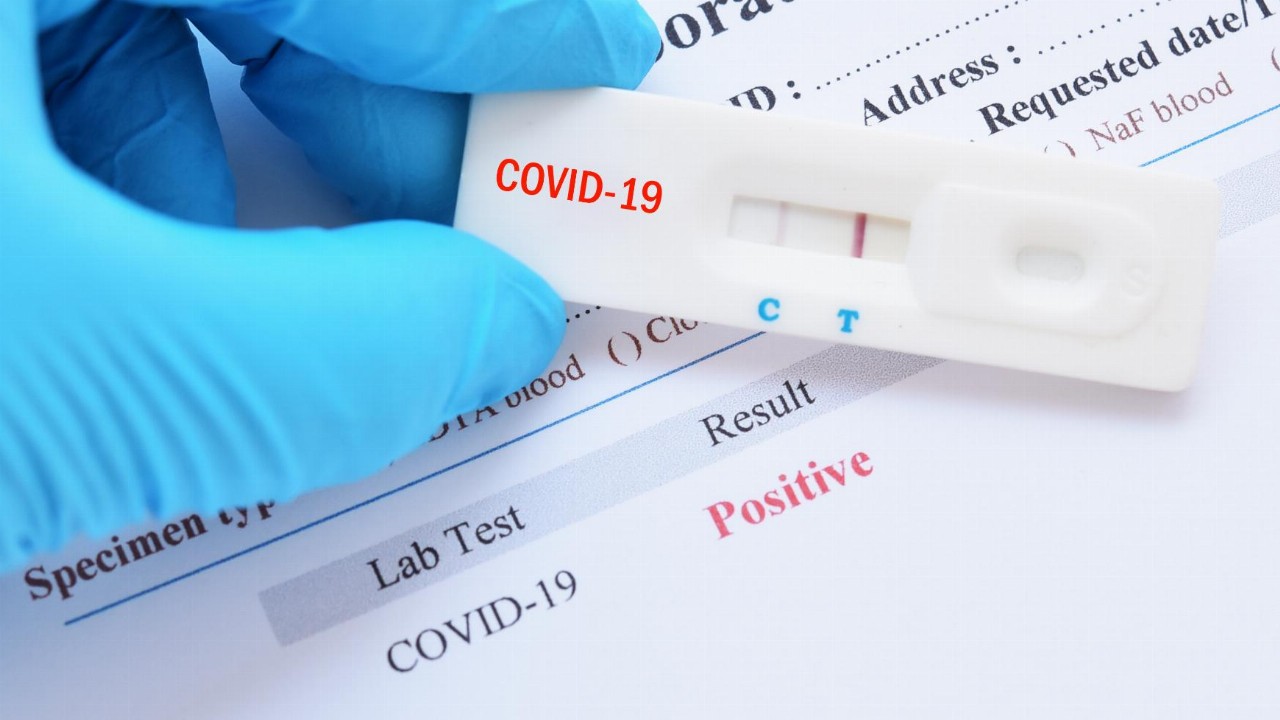
- In a new breakthrough, AnteoTech’s (ADO) COVID-19 test can now detect traces of the virus in saliva
- Recent lab testing using artificial mucus has shown the Antigen Rapid Test (ART) can detect the COVID-19 antigen in human saliva
- The company has declared a design freeze, meaning the product as it has met all the requirements to progress to the next stage of development
- AnteoTech will now validate the results in a clinical trial and is looking to collaborate with partners during this step
- The healthcare stock is on track for the clinical trial in December
- On the market this morning, AnteoTech is up 25.3 per cent and is trading at 9.9 cents per share
In a new breakthrough, AnteoTech’s (ADO) COVID-19 test can now detect traces of the virus in saliva.
Recent lab testing using artificial mucus has shown the Antigen Rapid Test (ART) can detect the COVID-19 antigen in human saliva.
As a result, the company has declared a design freeze for the product after completing its development process on time. Essentially, that means the ART design is now locked in so the company can continue developing the test.
The saliva test takes under 15 minutes and includes less than one minute of analysis time.
CEO Derek Thomson says this is an exciting development for ART.
“A saliva test is a less invasive and more comfortable test for patients, and only a few have been successfully developed and commercialised,” he said.
The company is now going to validate the results in a clinical trial and is looking to collaborate with partners for this step. Discussions for a clinical trial are underway with Victorian Infectious Diseases Reference Laboratory.
“If the results of the saliva trial are favourable and available in time, we are aiming to submit an approval application to the Therapeutic Goods Administration for a COVID-19 ART test capable of using either/or saliva and nasopharyngeal swab samples,” Derek told the market.
“Our development project for the COVID-19 Antigen Rapid Test is making excellent progress and we look forward to fulfilling an ongoing need for COVID-19 high sensitivity rapid testing in key global markets in 2021,” he added.
AnteoTech is on track to commence clinical trials for the COVID-19 ART test in December.
The global need for rapid COVID-19 testing has not eased despite promising signals from the vaccine industry. While a vaccine may be available to the majority of the population within the next year, it is unclear whether it will protect against the transmission of the virus or just protect against the illness.
On the market this morning, AnteoTech is up 25.3 per cent and is trading at 9.9 cents per share at 10:57 am AEDT.