- AVITA Medical has announced an institutional placement of $120 million to fund the pipeline development of clinical trials for its RECELL System
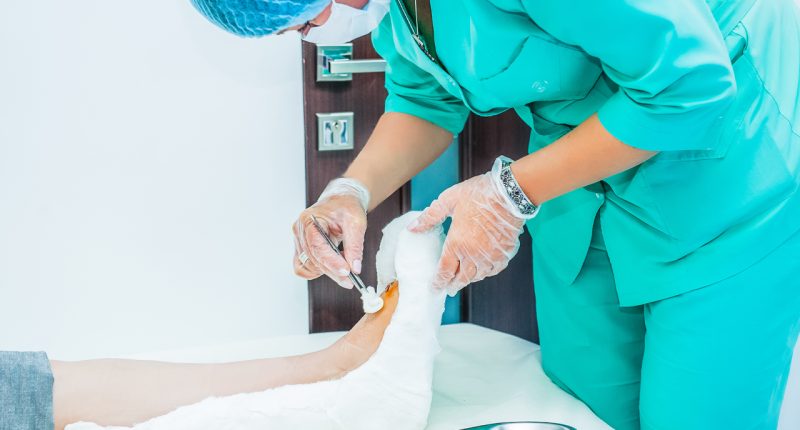
- RECELL is used to prepare Spray-On Skin Cells using a small amount of a patient’s own skin. This provides a new way to treat severe burns and repair skin tissue
- The burn treatment product is expected to target the unmet needs of thousands of burn patients in a more cost-effective way
- Despite the news, the company’s shares are down 6.52 per cent, with shares trading for 64.5 cents each
AVITA Medical has announced an institutional placement of A$120 million to fund the pipeline development of clinical trials and development projects.
The regenerative medicine company will also use the funds to continue its commercial growth strategy in the U.S.
The placement was managed by Bell Potter as well as Cowen and Lake Street Capital Markets who served as the financial advisor.
AVITA received commitments for $120 million at an issue price of 59 cents per fully paid ordinary share, representing a 7.2 per cent discount to the 30-day volume-weighted average price.
The placement will occur in a single tranche and issue 203,389,831 shares to international and Australian sophisticated investors.
“This financing reflects our shared excitement for, and strong confidence in, AVITA Medical’s myriad near-term growth opportunities,” CEO Dr Mike Perry said.
This funding will allow AVITA to progress its U.S pivotal trials of the RECELL System in soft tissue reconstruction and traumatic wounds, as well as early intervention in treating pediatric scald wounds.
“We are grateful for the confidence and conviction of the investors who have joined us in our mission to transform burn care with our revolutionary RECELL System and to promptly execute on our pipeline of add-on indications,” Dr Mike added.
RECELL was initially approved by the Food and Drug Administration (FDA) in September 2018.
However, the company plans to submit the next generation RECELL System to the FDA to facilitate access to the large outpatient market.
The RECELL System is TGA-registered in Australia and received CE-mark approval in Europe.
The RECELL System is used to prepare Spray-On Skin Cells using a small amount of a patient’s own skin, providing a new way to treat severe burns thus reducing the amount of donor skin required.
Data from randomised, controlled clinical trials conducted at U.S. Burn Centres and real-world use in over 8000 patients globally, reinforce the high demand for this product.
Additionally, AVITA is looking to secure marketing approval and reimbursement for the RECELL System in Japan, in collaboration with its partner COSMOTEC.
“Through diligent and focused execution on our highly de-risked pipeline of new indications, we have the opportunity to access the full potential of our innovative regenerative medicine platform to advance patient care that addresses unmet medical needs,” Dr Mike concluded.
Despite the news, the company’s shares are down 6.52 per cent, with shares trading for 64.5 cents each at 11:29 am AEDT.