- Brisbane-based Medtech developer ImpediMed (IPD) has received further clearance for its SOZO technology from the FDA
- This clearance enables ImpediMed to market SOZO for assessing patients at risk of protein-calorie malnutrition
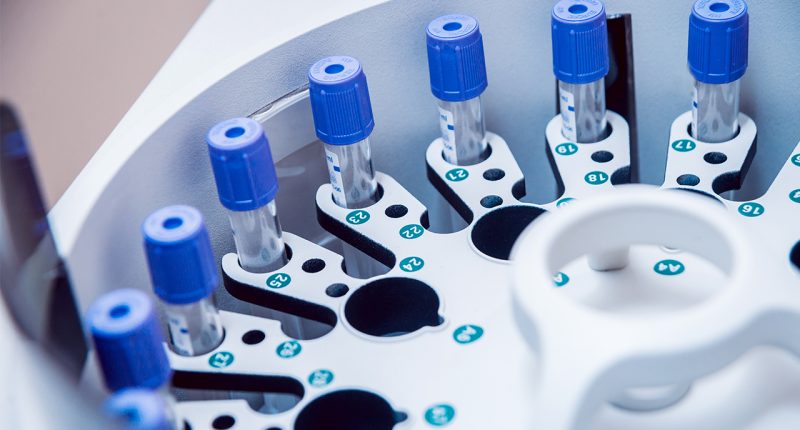
- SOZO delivers a precise snapshot of a patient’s fluid status and tissue composition in less than 30 seconds
- ImpediMed is up 11.1 per cent this morning with shares trading for 20 cents apiece
Brisbane-based Medtech developer ImpediMed (IPD) has received further clearance for its SOZO technology from the U.S. Food and Drug Administration (FDA).
The 510(k) clearance enables ImpediMed to market SOZO for assessing patients that are at risk of protein-calorie malnutrition (PCM).
It also tracks clinically relevant body composition parameters over time in healthy and unhealthy patient populations.
Specifically, the claims around PCM are to aid clinicians who are using Subjective Global Assessment (SGA) tools to assess patients at risk of PCM.
SGA tools, such as the American Society for Parental and Enteral Nutrition (A.S.P.E.N.) guidelines, define changes in physical attributes as assessment criteria for PCM in patients.
Weight, muscle and fat mass are tracked and reported by SOZO and can be used by clinicians to support their diagnosis and assessment and diagnosis of PCM.
PCM is a form of malnutrition, defined as a range of pathological conditions that arise from a lack of dietary protein and energy in varying proportions.
The disease is fairly common in both children and adults and accounts for six million deaths annually.
SOZO is the world’s most advanced, noninvasive bioimpedance spectroscopy (BIS) device.
The technology assesses patients with secondary lymphedema to deliver a precise snapshot of fluid status and tissue composition in less than 30 seconds.
A single SOZO reading allows clinicians across multiple specialities to provide individualised, proactive care that can help improve patient outcomes.
“We are pleased that this submission to the FDA included real-world evidence. This clearance will expand our clinical utility and footprint in the oncology space,” ImpediMed Managing Director and CEO Richard Carreon commented.
ImpediMed’s share price is up 11.1 per cent this morning with shares trading for 20 cents apiece at 11:31 am AEDT.