- IMEXHS (IME) has received a final device clarification to sell its Hiruko Essential Software in the United States
- PACS and RIS industry is expected to be worth US$3.9 billion by 2024
- IME will now be able to sell to the worlds largest PACS and RIS market
IMEXHS (IME) has received FDA approval to sell its Hiruko Essential Software in the United States.
IME is an Australian-based leading Software as a Service (SaaS) and ancillary solutions provider.
The company is known for its innovation in the imaging services market. it offers flexible and scalable imaging solutions via its Hiruko brand.
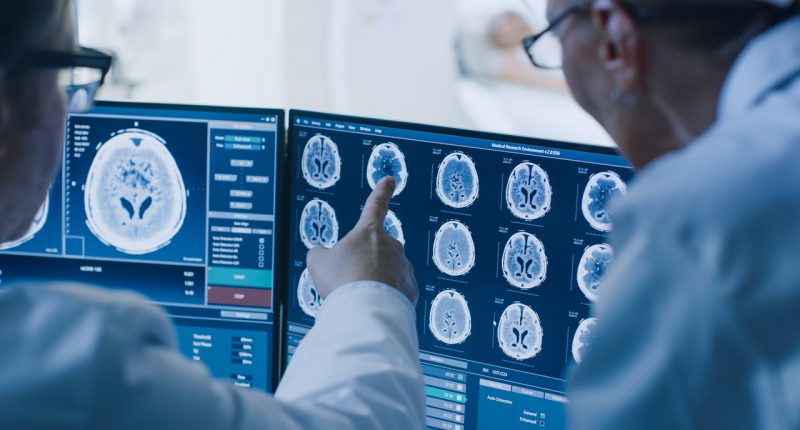
Hiruko Essential is a software-based device that receives digital images and data from various sources (e.g. Computed Tomography scanners, Magnetic Resonance scanners, ultrasound systems, computed & direct radiographic devices, etc.).
Images and data can be captured, stored, communicated, processed and displayed within the system and across computer networks.
The system boasts enhanced features such as a fully web-based voice recognition option and a zero footprint DICOM viewer.
Hiruko offers solutions for next generation Picture Archiving and Communications System (PACS) and integrated Radiology Imaging System (RIS) – both of which have a massive demand in the US.
According to a report by Transparency Market Research, the global PACS and RIS market was valued at US$2.2 billion in 2015 and is expected to be worth US$3.9 billion by 2024.
Now having received approval, IME can now market and supply its in-demand Hiruko Essential Software to the US.
PACS is a medical imaging technology which provides economical storage and convenient access.
RIS is a networked software system for managing medical imagery and associated data.
The American College of Radiologists noted there is 39,811 medical imaging facilities under its purview.
CEO Dr. German Arango is happy with the FDA clearance
“The receipt of the FDA clearance represents an important milestone in the history of the Company, and will allow IMEXHS to accelerate our soft launch in the US market,” he said.
On July 4, the company signed a five-year SaaS contract with Rad One PSC in Puerto Rico.
“The FDA clearance is also an important recognition for IMEXHS in Central and Latin America and the timing is particularly opportune given our recent appointment of a distributor in Brazil,” German said.
IMEXHS has gained 6.45 per cent today and is currently selling its shares at 6.6¢ per share.