- Cancer drug developer PharmAust (PAA) is continuing to progress with its MPL trial in dogs during the COVID-19 pandemic, albeit at a reduced pace
- The trial is testing the efficacy of Monepantel, or MPL, in dogs with naive B cell lymphoma, however, recruitment at some centres has been put on hold during the pandemic
- Based on historic human trial data and further studies, PharmAust has been working to improve the MPL tablets and is aiming to manufacture the refined tablet in the third quarter of 2020 for future human trials
- During the March quarter, the company’s subsidiary Epichem begun producing hand santiser to donate to healthcare and aged care agencies
- At the end of the quarter ending March 31, PharmAust had $3.23 million in cash, after receiving over $700,000 in research and development tax refunds
- Company shares are down 3.06 per cent and trading for 9.5 cents each
Cancer drug developer PharmAust (PAA) is continuing to progress with its MPL trial in dogs during the COVID-19 pandemic, albeit on a reduced scale.
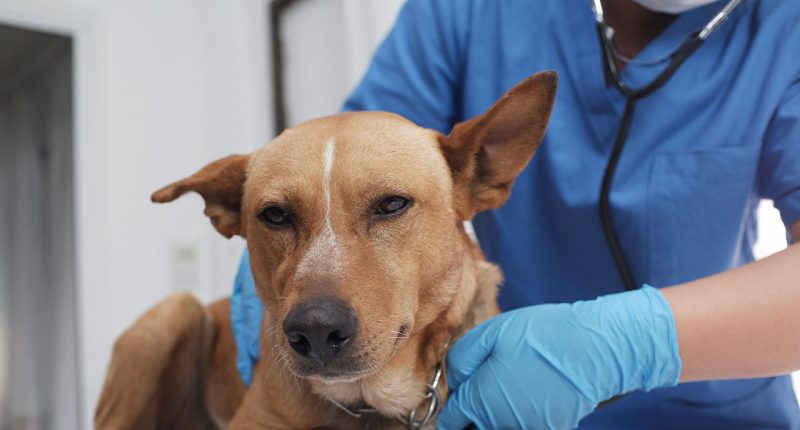
MPL in dogs
The trial is testing the efficacy of Monepantel, or MPL, in dogs with naive B cell lymphoma at five centres across Australia. The Brisbane Animal Hospital was engaged in March this year and was the latest site to come on board.
However, due to the risks posed during the pandemic, recruitment at the three sites in Melbourne and Sydney has been paused.
Any dogs already enrolled at those sites will continue until the completion of their 28-day trial period.
MPL in humans
Turning to human trial, PharmAust is continuing to progress towards further evaluations of MPL in humans. Data from a historic human trial of MPL has been submitted to a peer-review journal.
PharmAust has completed studies to increase the uptake of monepantel in the blood, reduce the number of tablets, and is also looking into changing tablet sizes to target optimal doses.
At present, the company is undertaking stability studies to test the shelf life of the tablets, successful results from which would support the relevant submissions to human trial ethics committees.
If all goes to plan, PharmAust plans to manufacture the refined MPL tablets in the third quarter of 2020 for future human trials.
Epichem
During the March quarter, the company’s wholly-owned subsidiary Epichem begun producing hand santiser at its laboratory in Western Australia.
The synthetic and medical chemistry business has been donating the hand sanitisers to aged care, disability, community, early learning and healthcare agencies including The Breast Cancer Research Centre and MercyCare’s Aged Care.
Cash in the kitty
As at March 31, 2020, PharmAust had $3.23 million in cash reserves up from $2.92 million at the end of the previous quarter.
The March quarter also saw the company receive $712,647 in research and development tax incentive refunds. These funds will be used to advance clinical trials in dogs and humans.
Company shares are down 3.06 per cent and trading for 9.5 cents each at 11:50 am AEST.