- Resonance Health has signed an agreement for US$1 million with a US NASDAQ listed pharmaceutical company for a clinical trial of its products
- Resonance specialises in development and delivery of non-invasive medical imaging software The clinical trial is for four years
- The client can cancel at anytime if trial is unsuccessful
Resonance Health has signed a deal for US$1 million with a U.S. NASDAQ listed pharmaceutical company for a clinical trial of its products.
The clinical trial will commence this month and will continue for four years. This could be extended if the clinical trial takes longer than expected.
Resonance is an Australian healthcare company that specialises in the development and delivery of non-invasive medical imaging software and services.
Monthly payments will be made to Resonance from August. The client at any time can cancel if it becomes clear the trial is unsuccessful.
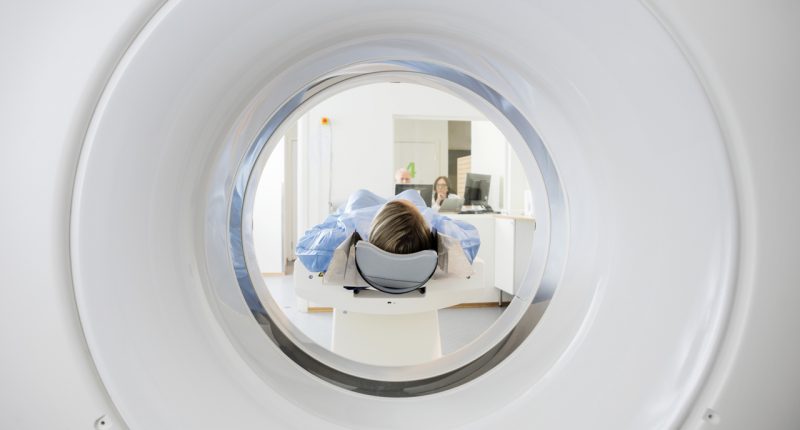
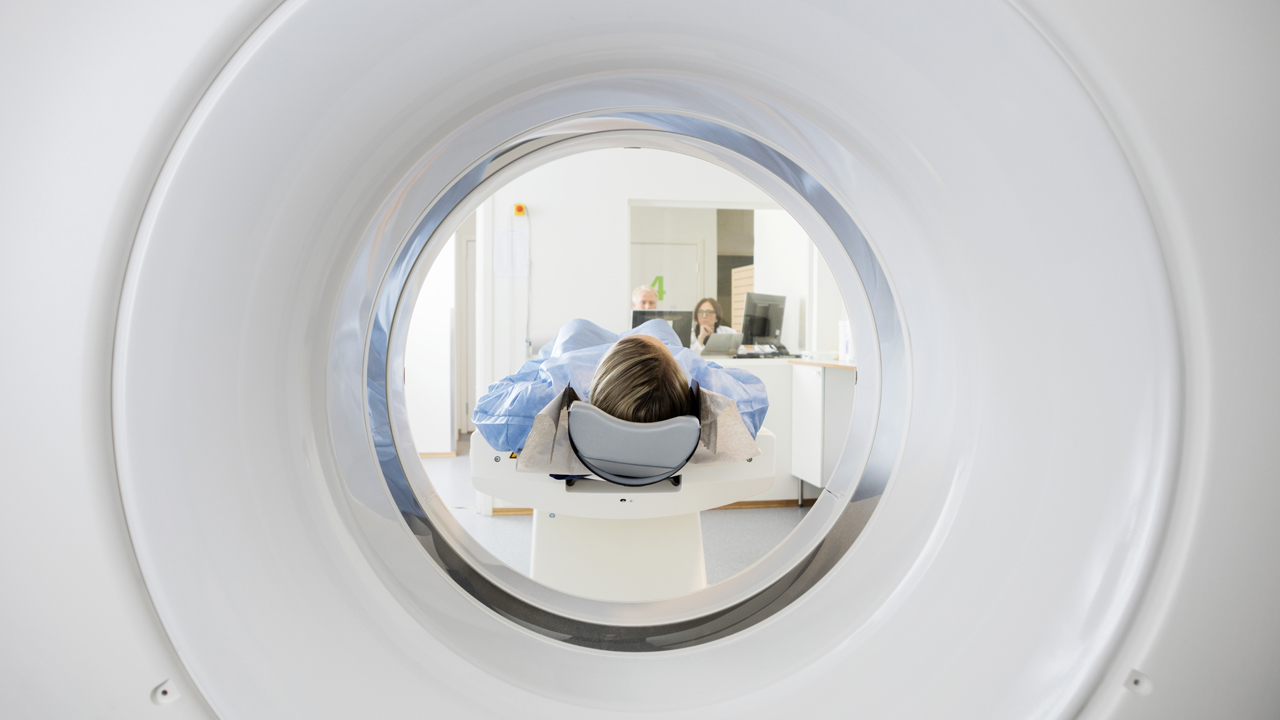
The products that will be sent over to the U.S. include FerriScan, Bone Marrow R2, Liver Volume, Spleen Iron, Spleen Volume and Spleen Phantom Pack supply and analysis.
One of the products, FerriScan, is an internationally recognised as the gold standard in liver iron concentration testing. It’s quick, easy and painless with a scan time of ten minutes.
FerriScan helps improve health outcomes of patients suffering from diseases such as thalassaemia (blood), sickle cell disease, haemochromatosis ( iron overload) and myelodysplastic syndrome (blood cells).
The company provides clinical trial services for pharmaceutical companies using imaging end points in their Phase II, III, and IV clinical trials.
A web based infrastructure enables image data and results to be transferred anywhere in the world to the lab in Australia.
Resonance’s head office is in Perth, Western Australia and has taken part in clinical trials across 25 countries in the past 10 years.
Resonance Health is up 8.33 per cent in the ASX today and is selling at 13 cents per share.