- Suda Pharmaceuticals Ltd (SUD) and Sanofi-Aventis Groupe have reached an agreement to conduct a feasibility study
- The study will investigate the feasibility of using one of Sanofi’s active ingredients in Suda’s OroMist Technology
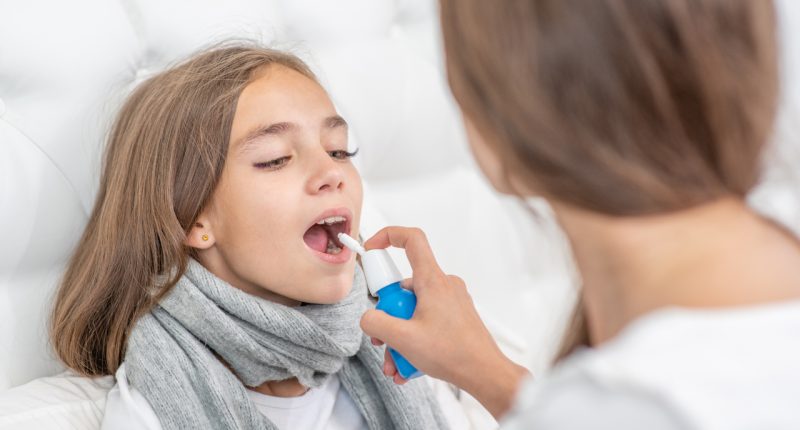
- This technology sprays a drug into the patients mouth, a method which increases absorbtion
- Suda will carry out the fully-funded feasibility study by the end of March, 2021
- Shares in Suda are unchanged, worth 6.6 cents apiece
Suda Pharmaceuticals (SUD) and Sanofi-Aventis Groupe have reached an agreement to conduct a feasibility study.
The study will research the use of one of Sanofi’s active ingredients in Suda’s OroMist Technology which sprays the drug into a patient’s mouth.
The feasibility agreement will see Sanofi fully-fund Suda to complete the study by March 31, 2021.
If the findings are promising, the two companies have indicated they may draw up further collaboration agreements.
Suda Pharmaceutical
Suda specialises in oro-mucosal drug administration. It’s OroMist technology delivers a drug to the oral cavity using either a pump or aerosol style spray.
The oro-mucosal membrane on the tongue, cheeks, palate and gums has a high concentration of blood vessels. This means that drugs delivered to the oral cavity can be quickly absorbed.
Furthermore, it oral absorption is less variable than through the digestive tract. There, it is impacted by several factors including stomach emptying time, effect of particular foods and enzymes in the gut.
Sanofi-Aventis Groupe
Sanofi is a global biopharmaceutical company with teams in over 100 countries.
These teams works in several areas including general medicine, vaccines, consumer health and specialty care. The specialty care units develops treatments for rare and complex diseases.
Shares in Suda are unchanged, worth 6.6 cents apiece at 12:06 AEDT.