- Admedus (AHZ) has received approval for the first in-human surgical aortic valve replacement trial for its ADAPT 3D aortic valve
- This trial comes after encouraging results from trials conducted on sheep
- Results from the human trial are expected to be received between quarter one and three in 2021
- Admedus has ended the day 4.35 per cent in the green with shares trading for 12 cents apiece
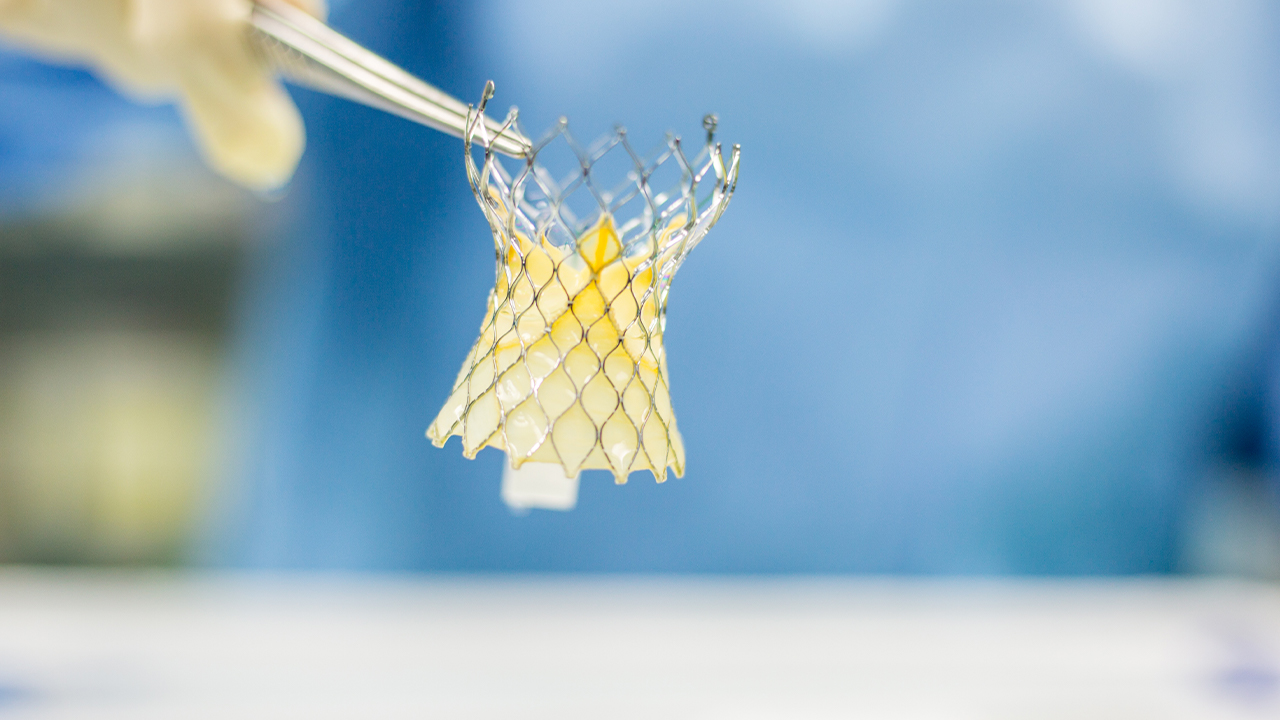
Admedus (AHZ) has received approval for the first in-human surgical aortic valve replacement (SAVR) trial of its ADAPT single-piece 3D aortic valve.
The trial will take place at the Leuven University Hospitals in Belgium and comes after the UZ Leuven Medical Ethics Committee approved the clinical trials protocol.
By 2023 the SAVR market is estimated to reach US$3 billion (AUD$4.38 billion) and more than 250,000 procedures were performed in Europe and North America in 2018.
Professor Bart Meuris, Cardiac Surgeon at the University Hospitals, is conducting the clinical trial which will be sponsored by Admedus’ subsidiary, Admedus Regen.
A total of 15 patients will enrol in the study and will be followed up for six months after receiving implantation of the ADAPT aortic valve.
Results from the human study are expected between Q1, 2021 and Q3, 2021.
ADAPT is a single-piece 3D aortic valve that is anatomically correct to a human aortic valve.
Tissue treated with ADAPT technology has been scientifically proven to more closely mimic the characteristics of normal human tissue which promotes a more tolerant immune response and improved tissue ingrowth.
According to Admedus, its ADAPT products are the only ones to have achieved over a 10-year period without calcification or degradation.
The first in-human trial follows Professor Meuris’ confidence in the prototype aortic valve after his recent investigation of the ADAPT valve in a sheep model.
Encouraged by the results, Professor Meuris expects that the ADAPT aortic valve will produce similar positive results in humans.
This study was performed on six juvenile sheep as they are the preferred model for bioprosthetic valve implants.
Results compare the implant’s function to that of a human valve, and track performance six months after the implantation. The tests revealed good and stable valve function and indicated the valve tissue was easy to handle during surgical implantation.
No material failure of the valve was observed, and echocardiography (heart ultrasound) showed low gradients and no significant regurgitation across the implanted valves.
“This endorsement of our ADAPT single-piece 3D aortic valve technology is an important next step to our development pathway,” CEO Wayne Paterson commented.
“The ADAPT single-piece 3D aortic valve has demonstrated excellent haemodynamics, both on the bench and in animal testing, and we expect to see that replicated in humans,” he added.
Admedus has ended the day 4.35 per cent in the green with shares trading for 12 cents apiece in a $67.94 million market cap.