- Exopharm (EX1) has announced the first dosing has occurred in the PLEXOVAL Phase 1 study using exosomes
- PLEXOVAL is a world-first study using a platelet-derived exosome product made from the company’s wound healing LEAP technology
- Exosomes show great promise in treating a number of age-related conditions as well as wound healing
- Currently though, the number of companies who can produce high quality exosome products is limited – this is where Exopharm comes in
- Despite the news, Exopharm is down 5 per cent and shares are trading for 28.5 cents each
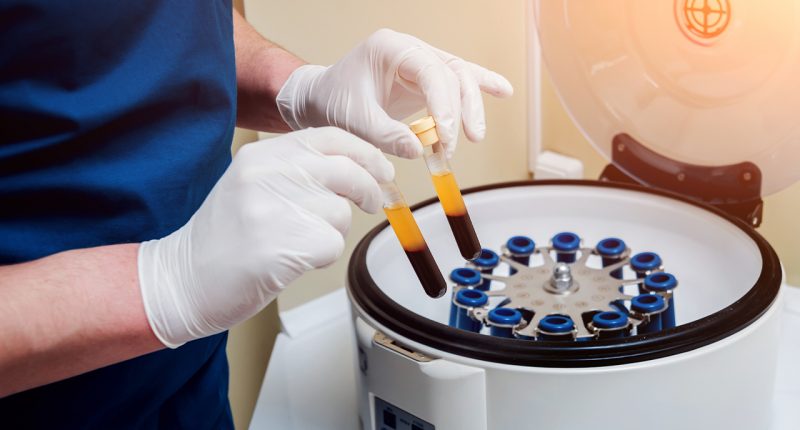
Exopharm (EX1) has announced the occurrence of the first dosing in the PLEXOVAL Phase I exosome wound healing study.
First dosing is an important milestone for Exopharm as a clinical stage company developing medicines based on exosomes.
“The first dosing of the PLEXOVAL study positions Exopharm as a clinical stage leader in the cell-free exosome field of regenerative medicine,” Exosopharm founder and CEO Dr Ian Dixon said.
“It is a great achievement for our team to test our exosome product in this Phase 1 study, which is a world first,” Ian added.
PLEXOVAL is a study using a cell-free, platelet-derived exosome product which has been made using the company’s LEAP Technology for healing and closing wounds.
The company began its this phase of the study in August last year. It marked the first human clinical trial using exosomes for wound healing and paved the way for Exopharm to become a world leader in exosome therapeutics for regenerative medicine.
Exosomes can reportedly bridge the gap in age-related medical conditions including problems with mobility and various sensory functions such as eye disease and hearing loss.
Unfortunately, the number of companies who can produce high quality exosome products at a clinical level is limited – this is where Exopharm comes in.
The company has the potential to satisfy a high demanded, unmet need in age-related medicines, wound healing and transplant rejection.
“Wounds and poor wound healing are medical problems affecting thousands of Australians every year,” Ian commented.
Exopharm noted that as we age, our ability to heal declines. Exosomes from platelets have been shown in animal studies to improve wound healing and reduce scarring.
As previously announced, dosing of Cohort 2 in the PLEXOVAL Study commences first and recruitment will now be rolled out.
Despite the news, Exopharm is down 5 per cent and shares are trading for 28.5 cents each at 11:28 am AEDT