- G Medical (GMV) has been granted TGA (Therapeutics Goods Administration) approval for its G Medical Holter Patch (GMP)
- The GMP streamlines and simplifies patient monitoring by providing real-time and continuous monitoring for up to two weeks
- It can measure heart rate, blood pressure, body temperature, heart rhythm, body position, and location
- It’s typically used for those in assisted living residences and hospitals
- The company is now looking for approval in other countries such as the U.S.
- G Medical is steady on the market this morning and shares are trading for 9.4 cents each
G Medical (GMV) has been granted TGA (Therapeutics Goods Administration) approval for its G Medical Holter Patch (GMP).
This marks an important milestone for the medical device company as its GMP now complies with all relevant medical and safety requirements for a Class IIa medical device in Australia.
A class IIa medical device is classified as low to medium risk and concern devices that are installed in the body in the short term, between 60 minutes and 30 days.
Examples include catheters, hearing aids, and blood transfusion tubes.
This TGA approval adds onto the already granted European CE Class II approval and G Medical is now seeking the green light for GMP in other countries, such as the U.S.
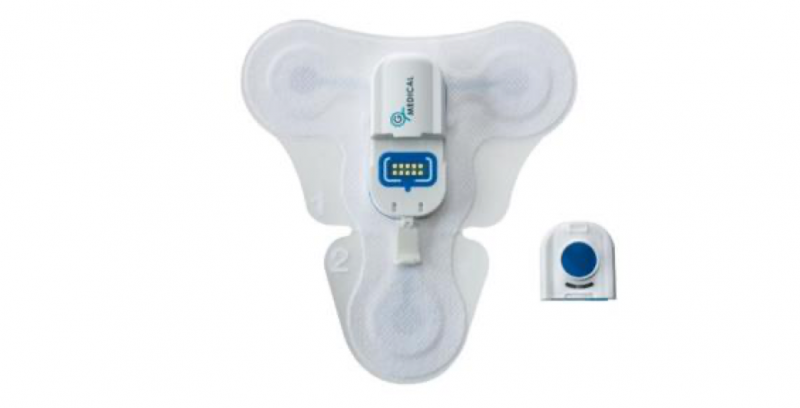
What is the GMP?
The GMP is a modular, easy-to-use clinical-grade solution used for monitoring patients throughout the healthcare lifecycle.
It’s typically used for those in assisted living residences and hospitals as it streamlines and simplifies patient monitoring by providing real-time and continuous monitoring for up to two weeks.
Sourced: G Medical
It can measure heart rate, blood pressure, body temperature, heart rhythm, body position, and location.
Patients can also mark a cardiac event, such as a heart attack, which will notify their doctor and relevant medical staff.
“TGA certification is a significant milestone for G Medical and demonstrates how the company’s suite of medical devices and telehealth technologies are relevant to the current environment,” CEO Dr Yacov Geva commented.
“The company continues to pursue regulatory approvals for both the GMP and the Prizma device in its target markets and looks forward to updating shareholders as they are granted,” he said.
G Medical is steady on the market this morning and shares are trading for 9.4 cents each at 10:21 am AEST.