- Invion (IVX) shares soar following its latest trial results, demonstrating a complete regression in tumours following in vivo treatment
- The company’s latest proof of concept studies used its drug INV043 to treat healthy mice that had been implanted with triple negative breast cancer (TNBC)
- Results showed a complete regression in the tumour and triggered an immune response preventing its recurrence, compared to the control group
- Further studies are ongoing, including a larger TNBC study and secondary study investigating the potential of the drug to treat metastatic disease
- Invion shares have sky-rocketed 112 per cent, trading at 2.8 cents at 1:00 pm AEDT
Invion (IVX) shares have soared following its latest trial results, demonstrating a complete regression in tumours following in vivo treatment.
The company’s latest proof of concept studies, undertaken by its research partner, used its drug, INV043, to treat immunocompetent mice that were implanted with triple negative breast cancer.
Triple negative breast cancer (TNBC) is an aggressive and metastatic tumour type that is known to be resistant to most chemotherapies, making them difficult to cure.
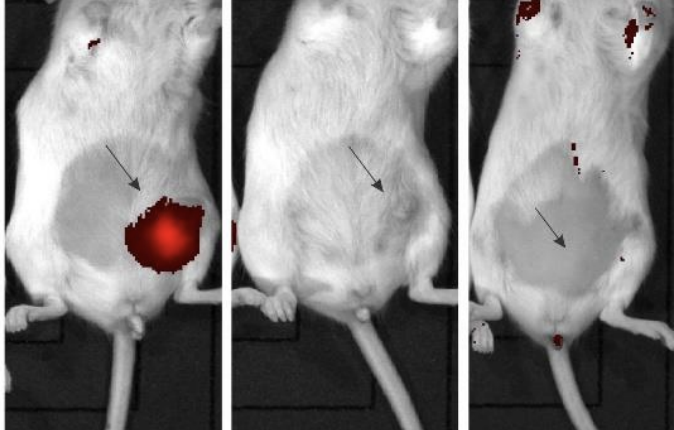
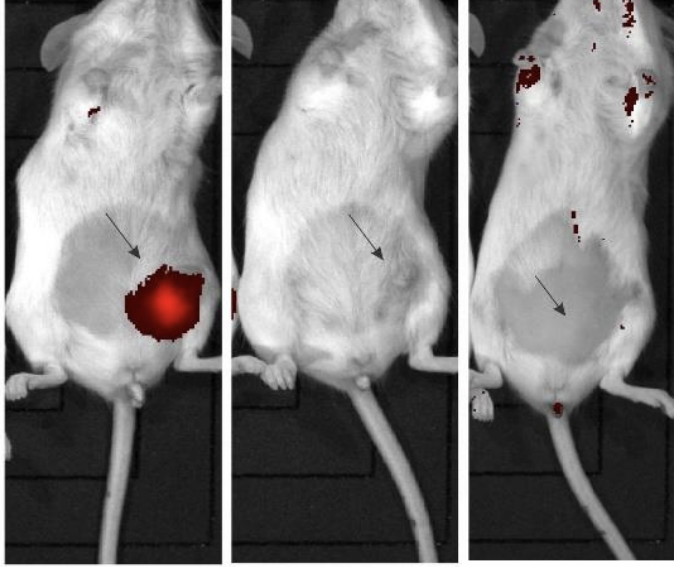
A treatment group of mice with established tumours had INV043 injected within the tumour. No adverse effects were observed, with the treatment then repeated nine days later.
Results from the study showed a complete regression in the tumour and appeared to trigger an immune response that prevents the recurrence of TNBC.
Moreover, the autopsy showed no apparent scarring or other indication of prior tumour presence, as well as no evidence of either primary tumour or metastatic spread.
In comparison, the control groups saw tumours rapidly enlarge up to more than 100 square millimetres by day 19, with autopsies showing the cancer had spread to the lungs and the abdominal fat pad.
Andrew Stephens, the Research Group Head at Invion’s research partner company, Hudson Institute, said INV043 is showing good promise as a potential treatment for TNBC, as well as a range of other hard to treat cancers.
“Not only did INV043 completely regress the primary tumour, but it also appears to have prevented the cancer from returning or spreading to other parts of the body,” Dr Stephens said.
“This sets it apart from other cancer treatments.”
Further studies are ongoing, including a larger TNBC study and a secondary study investigating the potential of INV043 to treat metastatic disease.
Invion shares have sky-rocketed 112 per cent, trading at 2.8 cents at 1:00 pm AEDT.