- Medlab’s NanoCBD product has received its first export order and deposit for 1500 units to Hong Kong
- This marks the third and newest cannabinoid product utilising Medlab’s NanoCelle delivery platform
- This is a significant step for global expansion with Australia and the US to potentially follow
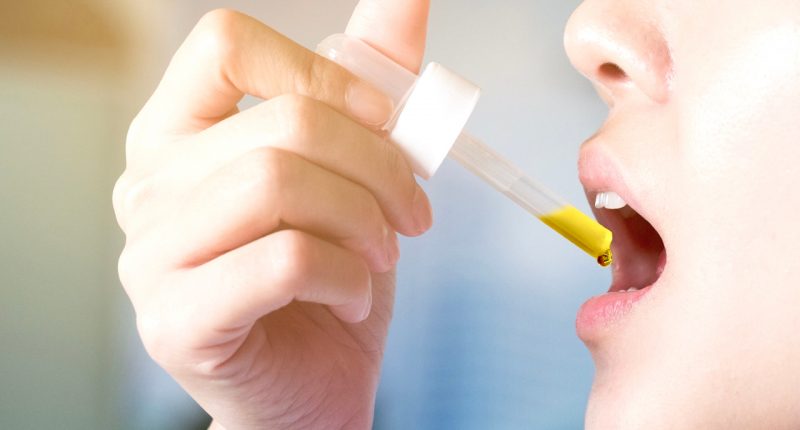
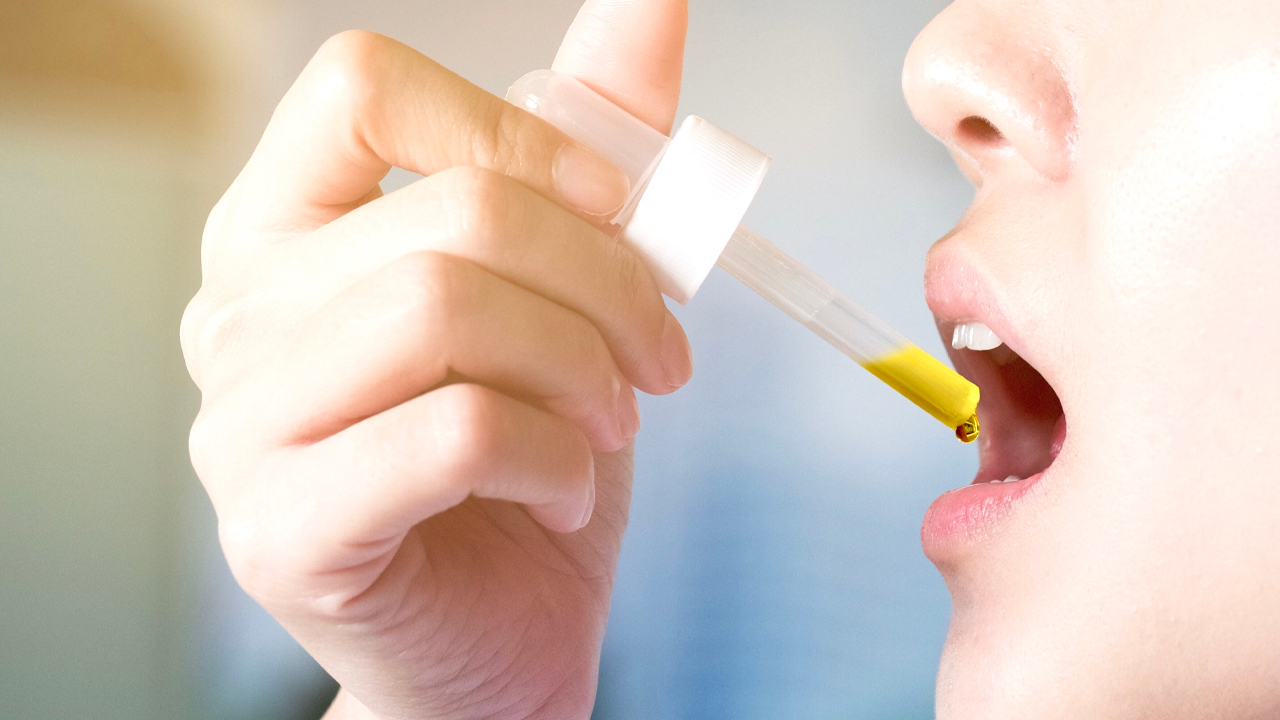
Medlab’s NanoCBD product has received its first export order and deposit for 1500 units to Hong Kong.
NanoCBD is a standardised formulation containing 16.67 mg/ml cannabinoid (CBD) from hemp, as an active ingredient, in a sub-micron spray applied to the inner lining of the cheek. It has been developed to treat inflammation and anxiety.
It is manufactured in a California facility that is U.S. Food and Drug Administration (FDA) approved and is certified by the Good Manufacturing Practice (GMP) system.
This marks the third and newest cannibinoid product utilising Medlab’s proprietary delivery platform, NanoCelle which delivers medicine in a tiny particle form in a spray.
Other products include NanaBis for treating cancer pain and potentially neuropathic pain, as well as NanaBidial for seizures, and nausea and vomiting during chemotherapy.
“NanoCBD is another example of the capabilities of Medlab’s patented NanoCelle delivery platform,” CEO Dr Sean Hall said.
NanoCBD allows for expanded commercial reach where hemp products have been legalised.
“The order is the start of global expansion for the product, with opportunities in the U.S. to follow. Once manufactured, NanoCBD will be available in Australia under SAS,” Dr Sean continued.
Medlab’s main objective is to achieve a Drug Registration with approved health claims, such as NanaBis and its Phase 2 Clinical Trial’s progress. Trial progress and pursuing drug registration will underwrite a global opportunity in pain management.
Additionally, the company is working with potential partners in U.S. countries that allow CBD from hemp as opposed to marijuana, for future market release.
Availability of the NanoCBD product is expected by Christmas 2019.