- ResApp has received CE Mark approval for ResAppDx-EU version 2 – the latest version of its smartphone app
- ResAppDx-EU version 2 uses machine learning algorithms that analyse a patient’s cough sounds to diagnose diseases
- The new approval is an extension of the already exisiting mark that ResApp received in August
ResApp has received CE Mark approval for ResAppDx-EU version 2, the latest version of the world’s first smartphone test for respiratory disease.
This new CE Mark approval is an extension of the already existing CE Mark that ResApp received in August. It adds the ability to test adults for lower respiratory tract disease, pneumonia, asthma exacerbation and chronic obstructive pulmonary disease.
The approval indicates that ResAppDx-EU version 2 meets the essential requirements for all the applicable European regulations as a class IIa medical device, and allows for its sales throughout the European Economic Area.
Most people will develop an acute respiratory tract infection every year and these infections are most commonly seen in primary care systems.
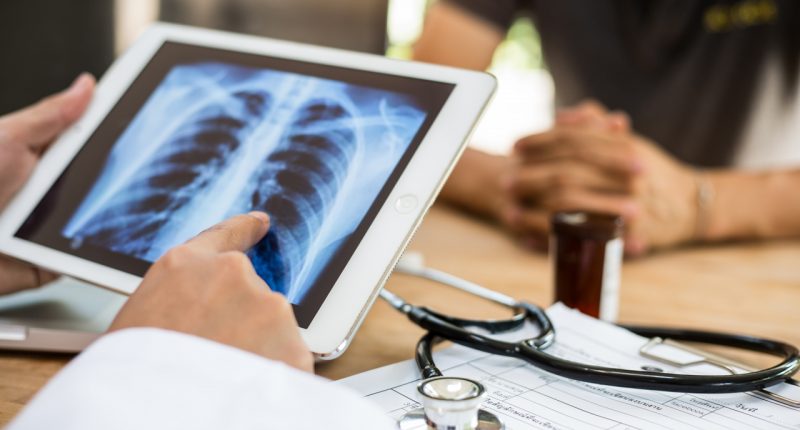
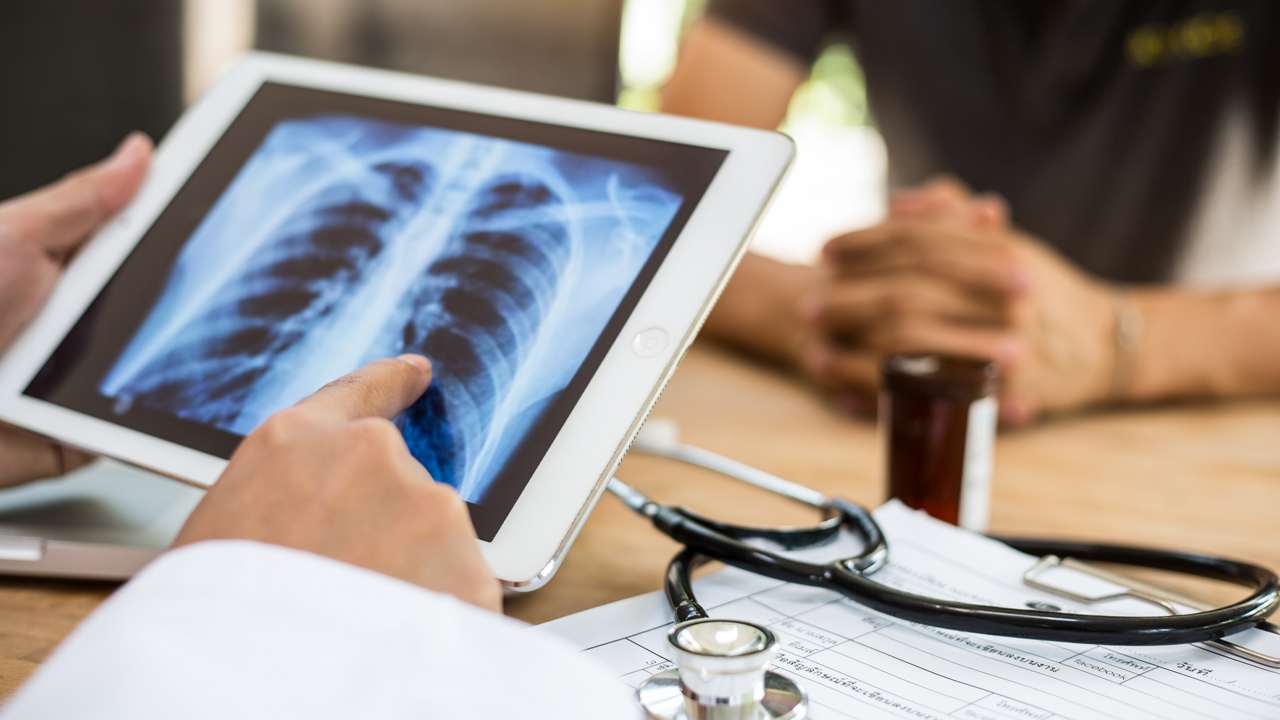
To diagnose acute respiratory diseases is a complex and lengthy process that combines clinical judgement with diagnostic tools such as a stethoscope, imaging and blood tests.
ResApp’s device uses machine learning algorithms that analyse a patient’s cough sounds to diagnose diseases.
The sounds of the coughs are then compared to those already in the system and the patient is diagnosed from there.
It is a software-only solution that runs on a smartphone and does not require any additional hardware or accessories.
“Achieving CE Mark approval allows us to commercialise our novel smartphone-based test for diagnosing acute respiratory disease in adults and children across the European Economic Area as well as in other regions that recognise the CE Mark,” company CEO Tony Keating said.
“A large percentage of general practitioner, emergency departments and telehealth consultations are for respiratory disease and now, for the first time, clinicians in Europe will have access to a fast and accurate point-of-care diagnostic test that will improve care and reduce costs for patients of all ages,” he said.
ResApp shares are up 4.55 per cent trading for 23 cents apiece at 11:23 am AEST. Its market cap is valued at $153.1 million.