- Over the past five weeks, Uscom (UCM) has received a material increase in demand for its USCOM 1A device in China
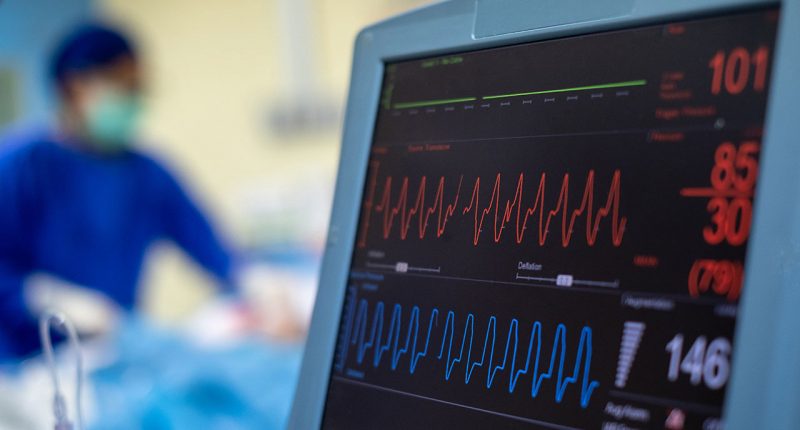
- The device is used to optimise the management of infectious diseases and accurately measures blood flow, cardiac output and stroke volume
- Due to the Coronavirus, unit orders for the first five weeks of 2020 are up 124 per cent compared to the first two months of 2019
- The company is planning to increase manufacturing output by approximately 121 per cent to meet the anticipated demand for the second half of the year
- Uscom has entered a trading halt, with shares last trading 52.4 per cent in the green for 48 cents apiece
In an update to the market today Uscom outlined the specific increase in USCOM 1A devices from 17 to 38 which is up 124 per cent increase from 2019 to 2020.
The company has also been reinstated to official quotation with shares down 7.29 per cent and trading for 44.5 cents apiece.
[This space has been left intentionally blank]
Over the past five weeks, Uscom (UCM) has received a material increase in demand for its USCOM 1A device in China.
Some devices are specifically being installed for the monitoring of hospital patients who have been diagnosed with Coronavirus.
This increase comes when just over a month ago the company received its third approval for the device.
The USCOM 1A haemodynamic monitor was developed to optimise the management of infectious diseases with most being deployed in the management of sepsis.
The device uses advanced doppler hemodynamics (evaluation of blood flow) to monitor cardiac blood flow.
It accurately measures blood flow, cardiac output and stroke volume across the aortic or pulmonary valve.
Measuring of stroke volume changes the way fluids in the body are managed and how sepsis, heart failure and hypertension are diagnosed.
Fluid remains one of the most challenging clinical interventions and inappropriate administration can have harmful outcomes.
USCOM 1A is improving management and saving lives in the neonatal, critical care, maternal, paediatric and emergency populations.
In response to the Coronavirus outbreak, The China National Health Commission released the new 5th Edition of the National Protocol for the Detection and Management of Coronavirus on February 5, 2020.
These protocols haemodynamic monitoring of severe and critically severe cases of the Coronavirus.
Uscom is planning to increase manufacturing output by approximately 121 per cent on 10-year average outputs to meet the anticipated demand for H2.
Unit orders for the first five weeks of 2020 are up 124 per cent compared to the first two months of 2019.
Despite the jump in sales, Uscom is unable to provide any more details regarding specific number due to the rapidly escalating situation.
However, it will continue to update the market with more information as it becomes available.
“The USCOM 1A is a specialised technology developed to simplify diagnosis and management of infectious diseases and is now being implemented widely in China under new National Government released Coronavirus Protocols to save the lives of the most seriously ill patients,” Executive Chairman, Associate Professor Rob Phillips said.
“[The Coronavirus] is a dangerous epidemic in the world’s most populous country, and the Government of China is acting decisively to limit the spread and impact of the disease by providing guidelines, equipment and personnel to most effectively care for the 1.4 billion people of China,” he added.
Uscom has been suspended from quotation with shares last trading 52.4 per cent in the green for 48 cents apiece.